Listen to “LIVING WITH SCHIZOPHRENIA IS NOT WHAT MOST PEOPLE THINK, ESPECIALLY SINCE IT’S NOT…” on Spreaker.
LIVING WITH SCHIZOPHRENIA IS NOT WHAT MOST PEOPLE THINK, ESPECIALLY SINCE IT’S NOT ALWAYS PORTRAYED ACCURATELY ON TV SHOWS AND IN THE MOVIES.
HOWEVER, WHILE THERE IS NO CURE FOR THE CONDITION, RECOVERY IS POSSIBLE FOR THE APPROXIMATELY 2.8 MILLION ADULTS WHO ARE LIVING WITH THIS CONDITION.
JOINING US NOW (DURING INVISIBLE DISABILITIES WEEK OCTOBER 15-21) IS CERTIFIED PSYCHIATRIST, DR. CHRISTOPH (CHRIS-toff) CORRELL (Coh-Rell) TO DISCUSS THE REALITIES FOR ADULTS LIVING WITH SCHIZOPHRENIA, THE CHANGING LANDSCAPE AND HOW TREATMENT CAN HELP THEM LIVE LIFE ON THEIR OWN TERMS.
POTENTIAL QUESTIONS
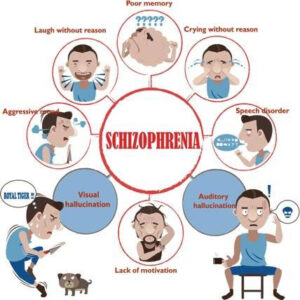
· Tell us about schizophrenia. What has your experience been working with adult patients living with schizophrenia?
· What are some of the challenges that adults living with schizophrenia face?
· What treatment options are available for those living with schizophrenia?
· What’s important to consider and talk to your healthcare provider about when deciding what the best course of treatment may be for you or a loved one?
· Any parting thoughts or advice you’d like to share with those who have been or have a loved one who lives with or has been recently diagnosed with schizophrenia?
BIO-Christoph Correll, M.D., is a board-certified general psychiatrist and child and adolescent psychiatrist based both in New York, USA, and Berlin, Germany. Dr. Correll is a professor of psychiatry and molecular medicine at the Zucker School of Medicine at Hofstra/Northwell, Hempstead, NY, and professor and chair of the Department of Child and Adolescent Psychiatry at Charité University Medicine in Berlin, Germany. He completed his medical studies at the Free University of Berlin in Germany and Dundee University Medical School in Scotland. Dr. Correll has authored and co-authored over 850 journal articles, including the recent poster “Efficacy and Safety of Paliperidone Palmitate 6-Month Formulation: A 3-Year Analysis in Adults with Schizophrenia.” He has also served on several expert consensus panels on the use of antipsychotics across a range of psychiatric disorders, and since 2014, the beginning of this metric, he has been listed every year by Clarivate/Web of Science as one of the “most influential scientific minds” and “top 1% cited scientists in the area of psychiatry” (https://hcr.clarivate.com).
Important Safey Information
INDICATIONS
INVEGA HAFYERA™ (6-month paliperidone palmitate) is a prescription medicine given by injection every 6 months by a healthcare professional and used to treat schizophrenia. INVEGA HAFYERA™ is used in adults who have been treated with either:
INVEGA SUSTENNA® (paliperidone palmitate) a 1-time-each-month paliperidone palmitate extended-release injectable suspension for at least 4 months
INVEGA TRINZA® (paliperidone palmitate) a 1-time-every-3-months paliperidone palmitate extended-release injectable suspension for at least 3 months
INVEGA TRINZA® is a prescription medicine given by injection every 3 months by a healthcare professional and used to treat schizophrenia. INVEGA TRINZA® is used in people who have been adequately treated with INVEGA SUSTENNA® for at least 4 months.
INVEGA SUSTENNA® is a prescription medicine given by injection by a healthcare professional.
INVEGA SUSTENNA® is used to treat schizophrenia in adults.
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about INVEGA HAFYERA™, INVEGA TRINZA® and INVEGA SUSTENNA®?
INVEGA HAFYERA™, INVEGA TRINZA® and INVEGA SUSTENNA® may cause serious side effects, including:
Increased risk of death in elderly people with dementia-related psychosis.
INVEGA HAFYERA™, INVEGA TRINZA® and INVEGA SUSTENNA® increase the risk of death in elderly people who have lost touch with reality (psychosis) due to confusion and memory loss (dementia). INVEGA HAFYERA™, INVEGA TRINZA® and INVEGA SUSTENNA® are not for the treatment of people with dementia-related psychosis.
Do not receive INVEGA HAFYERA™, INVEGA TRINZA® or INVEGA SUSTENNA® if you are allergic to paliperidone, paliperidone palmitate, risperidone, or any of the ingredients in INVEGA HAFYERA™, INVEGA TRINZA® or INVEGA SUSTENNA®. See the end of the Patient Information leaflet in the full Prescribing Information for a complete list of INVEGA HAFYERA™, INVEGA TRINZA® and
INVEGA SUSTENNA® ingredients.
Before you receive INVEGA HAFYERA™, INVEGA TRINZA® or INVEGA SUSTENNA®, tell your healthcare professional about all your medical conditions, including if you:
· have had Neuroleptic Malignant Syndrome (NMS)
· have or have had heart problems, including a heart attack, heart failure, abnormal heart rhythm, or long QT syndrome
· have or have had low levels of potassium or magnesium in your blood
· have or have had uncontrolled movements of your tongue, face, mouth, or jaw (tardive dyskinesia)
· have or have had kidney or liver problems
· have diabetes or have a family history of diabetes
· have Parkinson’s disease or a type of dementia called Lewy Body Dementia
· have had a low white blood cell count
· have had problems with dizziness or fainting or are being treated for high blood pressure
· have or have had seizures or epilepsy
· have any other medical conditions
· are pregnant or plan to become pregnant. It is not known if INVEGA HAFYERA™, INVEGA TRINZA® or INVEGA SUSTENNA® will harm your unborn baby
o If you become pregnant while taking INVEGA HAFYERA™, INVEGA TRINZA® or INVEGA SUSTENNA®, talk to your healthcare professional about registering with the National Pregnancy Registry for Atypical Antipsychotics. You can register by calling 1-866-961-2388 or visit http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry.
o Infants born to women who are treated with INVEGA HAFYERA™, INVEGA TRINZA® or INVEGA SUSTENNA® may experience symptoms such as tremors, irritability, excessive sleepiness, eye twitching, muscle spasms, decreased appetite, difficulty breathing, or abnormal movement of arms and legs. Let your healthcare professional know if these symptoms occur.
are breastfeeding or plan to breastfeed. INVEGA HAFYERA™, INVEGA TRINZA® or INVEGA SUSTENNA® can pass into your breast milk. Talk to your healthcare professional about the best way to feed your baby if you receive INVEGA HAFYERA™, INVEGA TRINZA® or INVEGA SUSTENNA®.
Tell your healthcare professional about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. INVEGA HAFYERA™, INVEGA TRINZA® and INVEGA SUSTENNA® may affect the way other medicines work, and other medicines may affect how INVEGA HAFYERA™, INVEGA TRINZA® and INVEGA SUSTENNA® works.
Your healthcare provider can tell you if it is safe to receive INVEGA HAFYERA™, INVEGA TRINZA® or INVEGA SUSTENNA® with your other medicines. Do not start or stop any medicines during treatment with INVEGA HAFYERA™, INVEGA TRINZA® or INVEGA SUSTENNA® without talking to your healthcare provider first. Know the medicines you take. Keep a list of them to show to your healthcare professional or pharmacist when you get a new medicine.
Patients (particularly the elderly) taking antipsychotics with certain health conditions or those on long-term therapy should be evaluated by their healthcare professional for the potential risk of falls.
How will I receive INVEGA HAFYERA™, INVEGA TRINZA® or INVEGA SUSTENNA®?
Follow your treatment schedule exactly as your healthcare provider tells you to.
Your healthcare provider will tell you how much you will receive and when you will receive it.
What should I avoid while receiving INVEGA HAFYERA™, INVEGA TRINZA® or
INVEGA SUSTENNA®?
INVEGA HAFYERA™, INVEGA TRINZA® and INVEGA SUSTENNA® may affect your ability to make decisions, think clearly, or react quickly. Do not drive, operate heavy machinery, or do other dangerous activities until you know how INVEGA HAFYERA™, INVEGA TRINZA® or INVEGA SUSTENNA® affects you.
Avoid getting overheated or dehydrated.
#schizophrenia #christophcorrell #invega #healthcare


 WHAT HAPPENS WHEN YOUR CHILD HAS A RARE AND DEBILITATING DISORDER CALLED RETT SYNDROME.
WHAT HAPPENS WHEN YOUR CHILD HAS A RARE AND DEBILITATING DISORDER CALLED RETT SYNDROME. 